Last Updated on September 23, 2023 by Aaron
Mozzarella is a traditional Italian cheese made with only a few ingredients — milk, rennet, salt, whey (optional), and critic acid.
To make the mozzarella taste good and stretchy, you’ll need all of them.
Interestingly, some of you are asking about the use of critic acid and rennet in the cheesemaking, or even possible to go without one of them.
I’ve elaborated that in my previous article where you can make feta without rennet.
The straight answer is yes. Many homemade mozzarella recipes use alternatives, which include lactic acid, vinegar, lemon juice, acetic acid, apple cider, and more.
Table of Contents
They are good to acid-set the cheese – which turns the milk into curds. But the problem is the added ingredients will usually give it a strong or sometimes weird aftertaste, for example, the apple cider.
So, it would be better to go with plain white vinegar.
What Does Acid Do in Mozzarella?
Milk contains roughly about 85% of water and 15% of solid content. Out of these solids, it has about 22% casein — a protein in milk, 38% lactose — a sugar, 30% fat, 5% ash, and 5% whey.
Citric acid and rennet are both chelating agents to coagulate or “glue” the caseins together.
They worked together to form curds but in a slightly different approach. Citric acid is also much cheaper than rennet. The commonly used animal-derived rennets also raised ethical concerns.
Citric Acid in Cheese Making is Common
Approximately 90% of the citric acids we use today were made from the fungi A. niger, which is not the citric fruit in common belief (1).
For that, some people may find these citric acids have an unpleasant aftertaste, so people sometimes use another ingredient as a replacement such as lemon juice or plain vinegar.
For some of the cheeses like Parmigiano Reggiano (aka Parmesan), no acid is added in the making. but they will use the whey starter from the batch one day before.
That is because of the presence of thermophilic lactic acid bacteria in the starter whey, where the bacteria will eat up the lactose and make some lactic acid naturally.
However, the acid volume wasn’t as much as citric acid used in making mozzarella, therefore, Parmigiano Reggiano curd settlement is more of a solid mass and not as soft, and as stretchy as mozzarella’s.
Similarly, in cottage cheese and quark, a starter is added for the bacteria to work.
The Function and Purpose of Citric Acid
The main function of citric acid in mozzarella is to adjust the pH — raising the acidity.
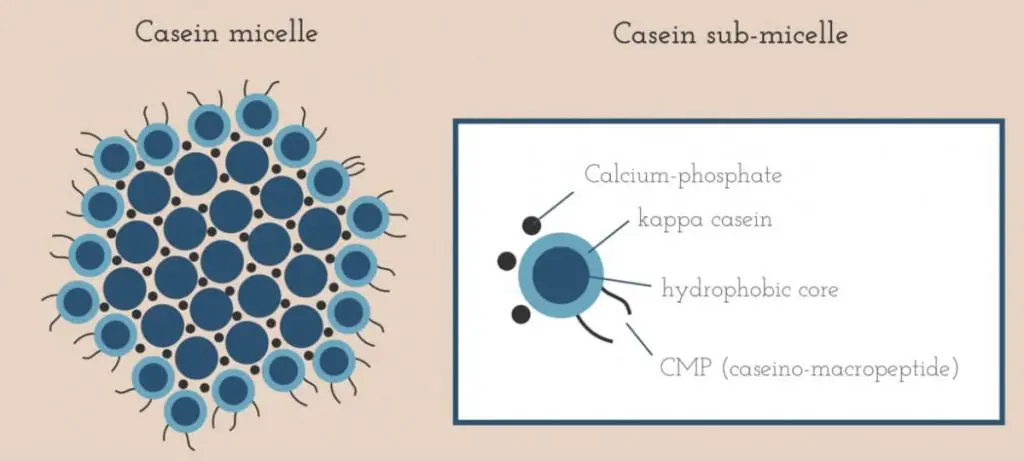
The outer layer of casein consists of a negatively charged substance called kappa casein, and because of that, all the negatively charged casein molecules repel and bounce off each other just like the same pole magnets under normal conditions — and therefore milky.
By adding citric acid,
It neutralizes the pH by providing more H+ ions (positive charges) to remove the negative charges on the casein— and therefore “glue” them together.
Citric acid removes/dissolves the calcium ion from the cheese (later drain out through the whey). The calcium ion is one key player binding the casein molecules together to form curd after the rennet was added.
Just like glue, too much calcium can hold the mozzarella too tight and less stretchy. So, it can be regulated by acidity.
Spongy cheese
High-fat milk can also make the cheese softer by creating spaces in between the caseins — thus spongy cheese. Heating the milk will destroy the structure of whey protein.
Then, the disrupted whey protein has both sticky ends which form bonds between the casein micelles under an acidic environment. That’s why heating will help curds aggregate well– if you ever heard about the stretched-curd or “pasta filata“.
Problems Without Citric Acid
If you plan to skip citric acid and use rennet only, it will work to an extent but you may find your mozzarella cheese has a bit of texture problem where the cheese could hardly coagulate well.
That’s because of the calcium regulation as explained above.
Think of citric acid as a medium to make the mozzarella curd shrink and soft.
Ideally, the cheesemakers will add a suitable volume of citric acid and rennet to get the best consistency.
Here is an illustration:
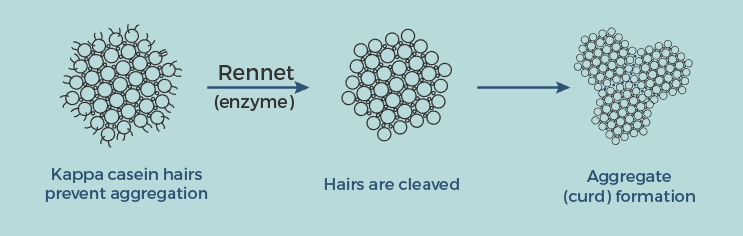
Why Do We Even Need Rennet? In the first place.
Rennet is derived naturally from the mammal stomach.
It contains a set of protein-cutting enzymes. For vegetarians, you can use gluten-free vegetable rennet. Enzymes like chymosin (learn chemistry here) and lipase will break down the caseins.
With that, rennet acted like a knife to cut off the Casein Macro-Peptide (CMP) tails which were located all over the surface of casein micelles.
See the image above. It results in the release of peptides (also the bitter taste). Later drained with whey.
Without the tails, casein micelles are then free to bind to each other with the help of calcium ions – which helps the curd formation.

